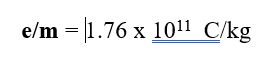Chemistry
STRUCTURE OF ATOM
CATHODE RAY EXPERIMENT
In this experiment, the 2 electrodes anode and cathode are taken in a Cathode ray tube. Anode is the positive terminal while the Cathode is the negative terminal. A high voltage of 10000V is applied in tube containing air.
Firstly, there are no visible changes in the cathode ray tube. The pressure is then lowered by using the vacuum pump. After the pressure is around 0.01atm, there is a red glow that can be seen behind the anode. Thomson concluded that this glow came from some rays that came from cathode. Thus he named these rays as cathode rays.
PROPERTIES OF CATHODE RAYS:
- They are negatively charged.
- They travel in straight line, i.e. they form a shadow of an object.
- They have mechanical effect; they revolve a paddle that lies in its path.
This shows that cathode rays have energy and they have particle nature.
These particles were called ELECTRONS.
- Nature of cathode rays does not depend on the gas taken.
- Mass of cathode rays is very small as compared to the mass of atom of gas taken.
All these properties show that the cathode rays were made up of small particles that were named as electrons and thus it was shown that ATOM was divisible.
WHY DISCHARGE TOOK PLACE?
If atom was divisible, it contained negatively charged particles called electrons. By applying high voltage, electrons came out of the atom and traveled to positive electrode (i.e. anode) and collided with cathode ray tube and glowed.
CATHODE RAYS: Cathode rays are high speed moving electron beams.
CHARACTERISTICS OF ELECTRON
- It is negatively charged particle with charge = -1.6×10-19 This is the smallest charge that exists and thus is called –1 relative charge.
- Its mass is 1/1840 of mass of a hydrogen atom.
Mass of 1 H atom = 1 amu = 1.67 x 10-27 kg
Thus mass of electron is negligible = 1/1840 amu
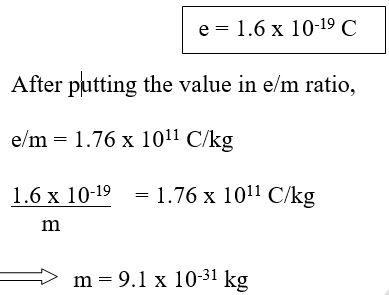
Absolute mass of electron = 9.1 x 10-31 kg
J.J. THOMSON’S EXPERIMENT
J.J. Thomson took a special type of discharge tube as shown above. It consists of cathode C, metal plate of anode A. Another metal plate D is there which has a small hole in it. There are 2 metal plates P1 & P2 as shown above. They can be connected to positive or negative electricity as required.
When there is no electric field applied at P1 & P2, cathode rays produced hit directly at E without any deflection.
When P1 is positive and P2 is negative, cathode rays get deflected to hit at E1 as cathode rays are negatively charged and get attracted towards positive terminal P1.
When P1 is negative and P2 is positive, rays get deflected towards E2.
After many experiments by taking different gases and applying different voltages, J.J. Thomson found the value of charge to mass ratio for cathode rays.
This value was constant for all gases and never changed. This value came around to be 2000 times more than the H atom.
The e/m for cathode rays = 2000 x e/m for H
Only 2 cases arise:
- e for cathode rays was 2000 times more
- m for cathode rays was 2000 times smaller.
Ultimately it was found that mass was 2000 times smaller for particles constituting cathode rays.
MILIKANS OIL DROP EXPERIMENT
After e/m ratio was found, Milikan performed his experiments on charges on oil drops. After detailed experiments, he found that the charge on oil drop was an integral multiple of 1.6 x 10-19 C. Thus he concluded that this was the smallest value of charge possible. This was called 1 e.
This mass was 1/1840 times of H atom mass. Thus it is negligible in comparison to other charges present in atom. Thus it was established that “Electron” was the constituting particle of cathode rays.
ANODE RAY EXPERIMENT
After electron was discovered, it was proposed that as atom is electrically neutral there should be some positive charges in the atom. More discharge tube experiments were conducted to establish this fact.
Here, perforated cathode was taken and same experiment was done as was in cathode rays. Here, a glow was seen at the back of cathode. These rays started from anode and passed through perforation in cathode and stroked on other side. After detailed experiments, it was concluded that these rays were positively charged. They were named as “anode rays.”
PROPERTIES OF ANODE RAYS
- Material nature
- Travel in straight line
- Positively charged
It was established these rays constituted of positively charged particles known as protons. Protons are H+ ions.
PROPERTIES OF PROTONS
- They are positively charged and have charge = +1.6 x 10-19 C = +1
relative charge.
- They have mass 1840 times that of electrons.
DISCOVERY OF NEUTRON
Mass of carbon is 12 amu. Carbon has 6 protons. Therefore, mass should have been 6 amu but mass was 12 amu. C also contains 6 electrons but mass of electrons was negligible.
Thus after experimental discovery, CHADWICK discovered another fundamental particle present in atom which had no charge but mass = 1 amu.
He named this particle as NEUTRON.
It was discovered that C contains 6 neutrons.
Total mass of carbon = mass of 6 protons + mass of 6 neutrons
= 6 + 6
= 12 amu.
HYDROGEN atom does not contain NEUTRON.
THOMSON’S MODEL OF AN ATOM
Size of an atom is around 10-10 m or 1A. He proposed that atom was a spherical substance with positive charge and electrons embedded on it.
He said that electrons were arranged such that they were embedded on the sphere.
RUTHERFORD’S MODEL OF AN ATOM
Rutherford conducted this experiment to find the atomic structure. The diagram is given above. The radioactive element gave out particles which struck the gold foil. He found that 99% of rays went straight without deflection. Only 1% rays deflected at some angle and around 1 in 1,00,000 deflected at 180o
FINDINGS OF RUTHERFORD
- He found that as only a fraction of rays deflected at 180o, so the entire mass of atom was concentrated at a small part of atom and named this as NUCLEUS of atom.
- As most of rays went straight without deflection, he proposed that most of the atom is empty.
- Deflection of such heavy particles (a particles) at large angles showed that the whole positive charge is concentrated at
- Electrons revolve around nucleus in orbits, the same way as in solar system.
DRAWBACK OF RUTHERFORD’S MODEL
He said that electrons revolve around the nucleus. Electrons are charged particles. We know for circular motion, particle is accelerated.
When charged particles accelerate, they release energy. Thus, the orbit of electrons should become shorter and at one time, electron should fall into the nucleus. But in reality it does not happen. Rutherford was not able to explain this phenomenon.
BOHR’S MODEL OF AN ATOM
To overcome the drawback of Rutherford, Bohr proposed his theory for structure of an atom.
FEATURES OF BOHR’S THEORY
1.) He said that rules governing subatomic particles were different from that of macroscopic objects.
2.) He said that electrons do not radiate energy when they move around nucleus in their orbits.
3.) All the orbits have discrete (specific) energy levels that are fixed.
- Electrons can jump from one orbit to another after gaining or releasing energy.
Whenever an electron gains energy, it moves from lower energy level to higher energy level.
If electron looses energy it radiates energy in form of electromagnetic waves. The wavelength emitted may be of some visible rays that can be seen distinctly.
- If very high energy is provided, electrons will move out of the atom. The cathode ray experiment showed that the electrons were ionized and struck the other side and then produced glow.
- Maximum number of electrons that can be present in a particular shell is constant.
DRAWBACK OF BOHR’S THEORY
All his theories were concentrated on H+ like ions. His theories could not work for larger atoms such as O, C etc
ELECTRONIC CONFIGURATION
- a) According to Bohr, maximum number of electrons in an orbit is fixed.
He named orbits using alphabets:
K – 1st shell
L – 2nd shell
M – 3rd shell
N – 4th shell
Maximum number of electrons that can be accommodated in a shell is given by 2n2, where ‘n’ is the shell number.
- b) Maximum number of electrons in the outermost shell of atom can not be more than 8
- c) Number of electrons in outermost shell can not be more than 2 untill the previous shell is filled according to 2n2
ATOMIC NUMBER AND MASS NUMBER
ATOMIC NUMBER (Z) = Number of proton
= Number of electrons (for a neutral atom)
MASS NUMBER (A) = number of protons + number of neutrons
VALENCE ELECTRONS
Valence electrons are electrons present in the outermost/valence shell. These electrons determine the chemical properties of any atom.
VALENCY
It is the combining capacity of any atom. It is either equal to valence electrons or 8-valence electrons (for more than 4 valence electrons).
ISOTOPES
Elements having same atomic number but different mass number are called ISOTOPES. They always have different number of neutrons. For example there are three isotopes of carbon- 6C12, 6C13, 6C14.
AVERAGE ATOMIC MASS
When an element is found in isotopic forms then it mass is given as the average mass of its all isotopic forms in relation to their abundance in the nature. It is calculated with the following formula :-
Average atomic mass of an element = mass of first isotopic form X its abundance (in %) in the nature/100 + mass of second isotopic form X its abundance in the nature/100 + …………+ mass of nth isotopic form X abundance in the nature/100
(where n is the number of isotopes of the element)
For example Chlorine is found in two isotopic forms – 17Cl35 and 17Cl37 which occur 75% and 25% in the nature. thus it mass is given as –
The average atomic = 35 X 75 + 37 X 25 = 35.5u
mass of Chlorine 100 100
APPLICATIONS OF ISOTOPES
- An isotope of cobalt is used in the treatment of cancer.
- An isotope of iodine is used in the treatment of goiter.
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of carbon (C-14) is used in age determination of dead remains of living things.
- An isotope of hydrogen(Deuterium) is used in reactors in the form of heavy water(D2O)
ISOBARS
Isobars are defined as the atoms of different elements having different atomic numbers but the same mass number. For example : 18Ar40 (Argon) , 19K40 (potassium) and 20Ca40(Calcium) are isobars to each other.